Johns Hopkins University SPORE of Ovarian Cancer
in collaboration with the University of Pennsylvania
Principal Investigator: Ie-Ming Shih, MD, PhD
Co-Principal Investigators:
Ronny Drapkin, MD, PhD (University of Pennsylvania)
D
Ovarian cancer is one of the most aggressive cancers in women in the United States and a major cause of cancer morbidity and mortality. This Johns Hopkins-University of Pennsylvania Ovarian Cancer SPORE application focuses on reducing ovarian cancer incidence and mortality by translating new laboratory research discoveries made in our institution into improvements in ovarian cancer detection and treatment. This highly translational program contains four hypothesis-driven Research Projects, three Core Resources, the Career Enhancement Program, and the Developmental Research Program. The objective of Project 1 is to determine whether detection of tumor cells from liquid-based cervical fluid specimens, endometrial cavity brushing and/or circulating tumor DNA (ctDNA) from blood can identify early and low-volume ovarian high-grade serous carcinoma (HGSC), the most common type of ovarian carcinoma, or its precursor lesion, serous tubal intraepithelial carcinoma. The goal of Project 2 and Project 3 is to provide critical preclinical and early clinical data for developing more effective combined therapy to treat advanced ovarian HGSC, especially for recurrent diseases. Specifically, Project 2is to optimize synthetic lethality in high-grade ovarian serous ovarian cancer by using ATR inhibitor and PARP inhibitor. Project 3 based on a recent discovery made by our team proposes to apply BET inhibitors for overcoming platinum resistance. Project 4 aims to determine that inhibition of Spleen Tyrosine Kinase (SYK) activity sensitizes ovarian cancer cells to the cytotoxic effect of paclitaxel, and determine if SYK inhibitor represents a promising new agent to be combined with (weekly) paclitaxel for the treatment of advanced ovarian cancer.
These Projects are supported by an Administrative Core, a Biorepository/Pathology Core, and a Biostatistics Core. Finally, the Career Enhancement and Developmental Research Programs comprise pipelines of human capital and innovative ideas, respectively, which will fuel future SPORE advances. This application is strongly supported by institutional commitment to ensure its success.
Detached cancer cells like those in the circulation, peritoneal fluid and ascites are characterized by "microtentacles" on the cell surface which are microtubule-based membrane protrusions. Micortentacle formation may promote seeding of ovarian cancer cells within the peritoneal cavity. We are studying how ovarian cancer-specific signal transduction pathways affect the genesis and dynamics of microtentacles and propose a new intervention to abort those protrusions and suppress intraperitoneal dissemination of ovarian cancer.
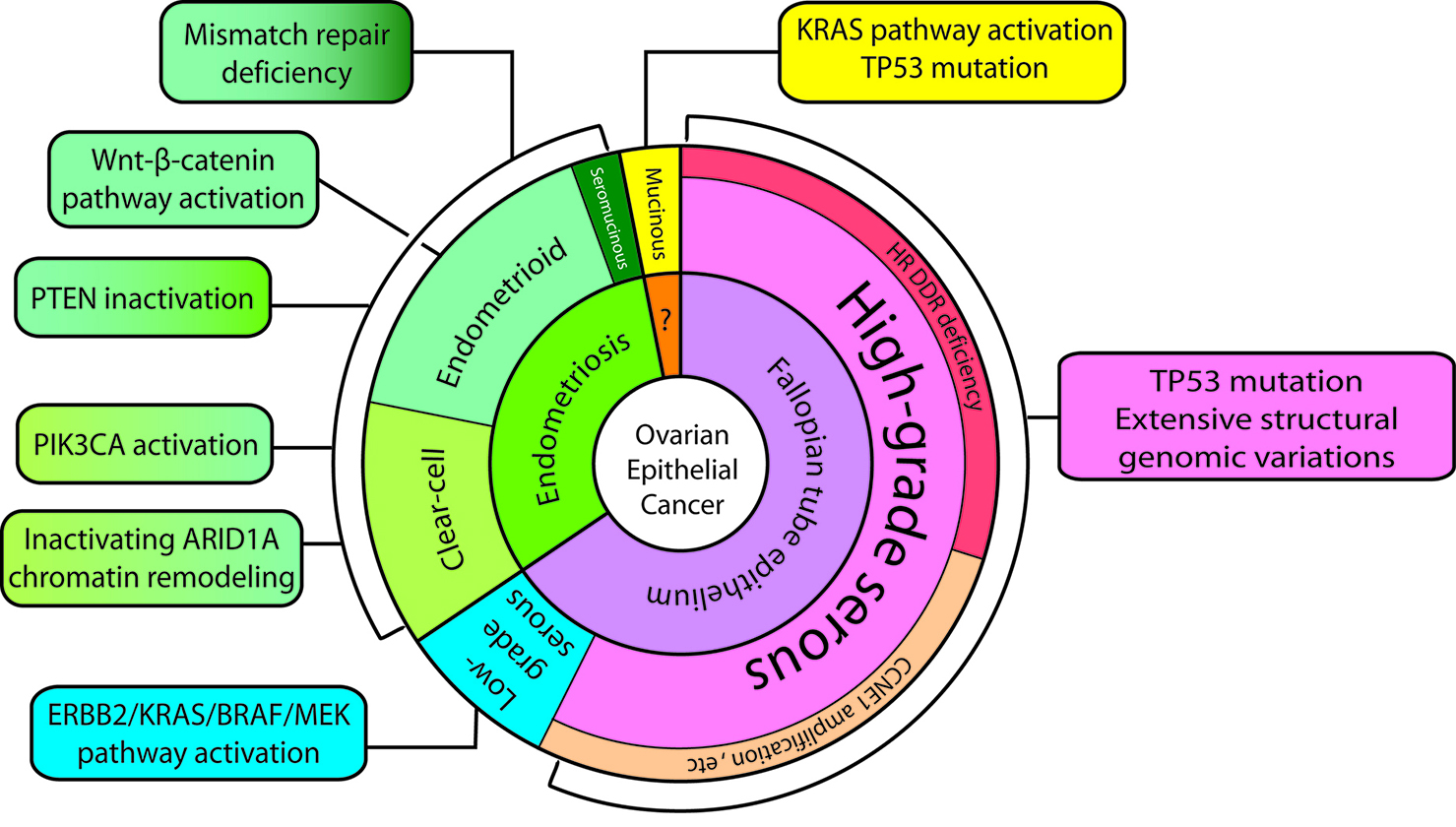
Ovarian cancer is the most lethal gynecologic cancer and the fifth most common cause of cancer-related death for women in the United States (1) (Ovarian Cancer Statistics). Nearly 22,000 women in the U.S. are diagnosed with ovarian cancer annually, and approximately 14,200 women die each year from this disease (based on Cancer Facts & Figures). Although recent advances in treatment have increased the disease free interval, overall survival of ovarian cancer has only slightly improved over the last 50 years. Our understanding of carcinogenesis and tumor classification in the past is largely based on studying morphology, but the importance of molecular pathways in characterizing carcinogenesis is becoming increasingly apparent. The new model (Figure 1) is intended to integrate the existing histopathologic classification with the emerging molecular findings so as to provide a bridge to the future. The model now divides type I tumors into three groups: 1) endometriosis-related tumors which include endometrioid, clear cell (Figure 2, 3) and seromucinous carcinomas (Supplemental Figure), 2) low-grade serous carcinomas and 3) mucinous carcinomas and malignant Brenner tumors (Table 1). Type II tumors are composed, for the most part, of high-grade serous carcinomas, undifferentiated carcinomas, carcinosarcomas and primary peritoneal carcinomas. The high-grade serous carcinomas can be subdivided into morphologic and molecular subtypes, further underscoring the heterogeneity of epithelial ovarian carcinoma. Almost invariably, type II ovarian cancers are highly aggressive and present as advanced stage diseases including tumor ascites, metastasis, carcinomatosis and diffuse involvement of omentum (so called "omental caking"). Type II carcinomas exhibit a high proliferative activity and are thus sensitive to carboplatin/paclitaxel combination therapy in the beginning, while most type I carcinomas show a very low proliferative activity and are refractory to conventional chemotherapy. Frequent mitoses can be found in type II carcinoma bur rarely in type I neoplasms, and type II tumor cells contain abnormal mitoses, highlighting the underlying genomic instability. Type I ovarian cancers always develop in a stepwise fashion with intermediate lesions called "borderline tumors" before they become an invasive carcinoma, and this process may take several years (unlike type II tumors which develop rapidly). A morphological continuum of different stages of tumor progression can be sometimes detected in surgical specimens (see the Example). An example of Ki-67 stained low-grade (type I) and high-grade (type II) serous carcinomas is shown in the Supplemental Figure. Type I tumors develop from benign extraovarian lesions that implant on the ovary and which can then undergo malignant transformation. They behave in an indolent fashion because they are generally confined to the ovary at diagnosis. Many of ovarian clear cell, endometrioid and seromucinous carcinomas are believed to derive from ovarian endometriotic cysts. An example of stage I clear cell carcinoma arising from a pre-existing endometriotic cyst of the ovary is shown (Histology, Ultrasound). Ovarian mucinous carcinomas grow slowly and are most often stage I diseases at the time of presentation. Their origins are unclear but a recent study using molecular analysis reveals different cell of origins in mucinous carcinomas (Figure 1). In contrast, many type II carcinomas develop from an intraepithelial carcinoma in the fallopian tube and, as a result, disseminate as carcinomas that involve the ovary as well as extraovarian sites, which probably accounts for their clinically aggressive behavior. In contrast to type II carcinomas, type I carcinomas are relatively genetically stable, notably lacking TP53 mutations and BRCA-related abnormalities. In contrast, type II ovarian cancers almost always harbor TP53 mutations which are a defining feature of high-grade serous carcinomas. The molecular genetic studies underscore the heterogeneity of ovarian cancer, which is a family of related but distinct tumors with significant differences in molecular genetic features, clinicopathologic characteristics and behavior that require different approaches to management. The dualistic model highlights these differences between type I and type II tumors which, it can be argued, describe different groups of diseases. The histologic features of various ovarian carcinomas are shown in this Supplemental Figure and the aberrations in molecular pathways specific to different histologic types of ovarian cancer are summarized in another Supplemental Figure. Of note, although ovarian high-grade serous (type II) carcinoma shares many features with uterine serous carcinoma, both are characterized by different molecular alterations (Supplemental Figure).
Figure 1. The revised dualistic model in the pathogenesis of ovarian epithelial cancer. Type I carcinomas are comprised of low-grade serous, clear cell, endometrioid, and mucinous carcinomas. Seromucinous carcinomas and malignant Brenner tumors are rare and not shown. Type II carcinomas are largely composed of high-grade serous carcinomas. Carcinosarcoma and undifferentiated carcinoma are relatively uncommon and not illustrated. The areas in individual histotypes reflect their relative prevalence. The inner circle indicates the likely cell of origin of the different Type I and Type II neoplasms. The origin in mucinous carcinomas is not well established and is discussed in the text. The molecular pathway alterations that characterize each tumor subtype are summarized in the square boxes. Some of the pathway abnormalities are shared by some tumor types and they are shown in two-color fill in boxes.
Gross picture of an ovarian high-grade serous carcinoma involving small intestine. High-grade serous carcinoma is the most common and aggressive type of ovarian epithelial carcinoma. It is always diagnosed at advanced stage (like this case illustrated). The 5-year survival rate is less than 40%.
It has been well accepted that there is an unmet need in ovarian cancer research to develop a better tool for the detection and monitoring of this disease and to deliver more effective treatment regimens for women suffering from ovarian cancer. There are at least two obstacles that impede efforts to improve clinical outcome in ovarian cancer: 1) lack of sensitive and specific methods for its detection at an early stage and 2) lack of effective treatments for patients with advanced stage disease. These barriers are also highlighted by an international Ovarian Cancer Research Consortium in a recent review article (2). Both barriers have been largely due to limited understanding of disease etiology, tumor immune microenvironment, or the mechanism of disease becoming refractory to treatment. Therefore, historically, many detection and treatment strategies have been empiric rather than based on rational design of the products. Advances in our molecular biology techniques now offer hope for developing new methods of ovarian cancer early detection, and similarly, recent advances in our understanding of how the immune system can be unleashed to destroy tumor cells offers new hope for improving current treatment beyond the use of cytotoxic chemotherapy. For over a decade, ovarian cancer investigators at the Johns Hopkins University (JHU) have focused their research efforts on the pathogenesis pathways of ovarian cancer, including shifting the emphasis of disease origin from the ovarian surface epithelium to the fallopian tube epithelium, identifying negative immune regulators (checkpoints) in the tumor microenvironment, and identifying a kinase signaling pathway involved in the acquired chemoresistance. Accordingly, the JHU ovarian cancer program is established based on the advancements of these scientific endeavors, with the intent to provide rationale-based detection and treatment opportunities and ultimately to improve disease outcome for ovarian cancer patients.
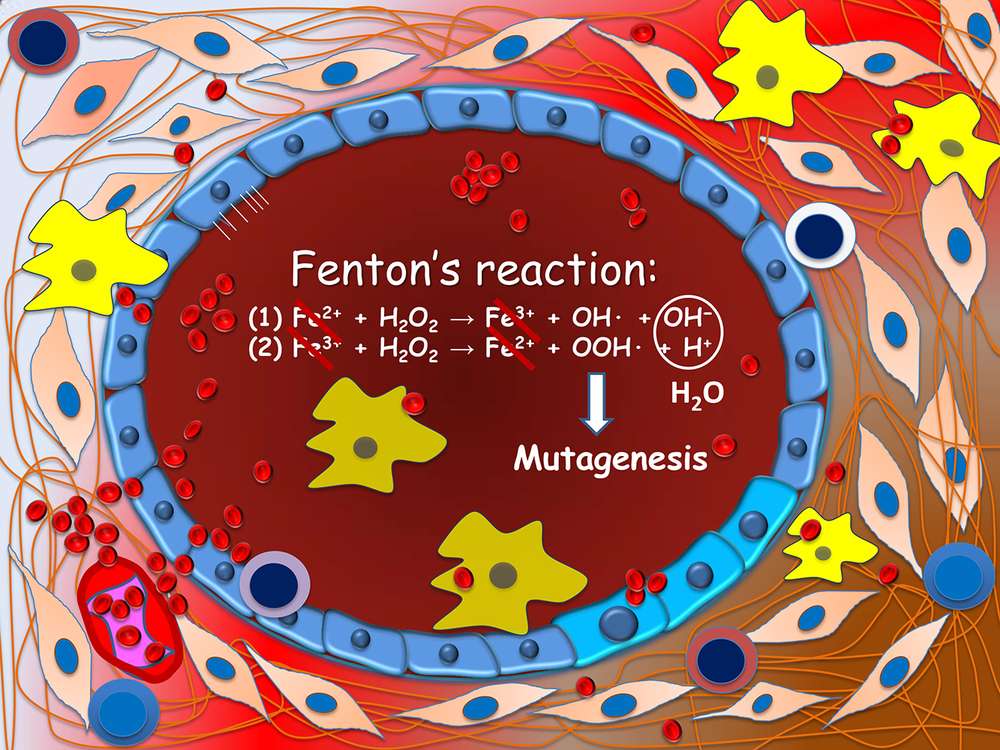
Figure 2. Carcinogenesis of endometriosis-associated ovarian neoplasms. Ovarian endometrioma (depicted) is lined by a single layer of endometriotic epithelium. Repeated hemorrhage as occurs during menstruation creates a highly inflammatory microenvironment. Reactive oxygen species are produced through the Fenton's reaction in the presence of abundance ferrous iron. These free radicals are highly genotoxic and may damage DNA of epithelial cells, a prerequisite step leading to gene mutations.
Figure 3. The histologic features of an ovarian clear cell carcinoma (H&E, left panel). An identical PIK3CA somatic mutation is detected in both clear cell carcinoma and the adjacent endometriotic cyst epithelium, suggesting that endometriotic cyst epithelium is the precursor of clear cell carcinoma.
Task 1: The need to develop better detection strategies – Approaches for early diagnosis of cancer have been effective in reducing the morbidity and mortality of several types of neoplastic diseases. Notably, Pap smear screening has significantly reduced the incidence and mortality of cervical cancer in women. However, early detection of ovarian cancer has been a monumental challenge. The screening strategies that have been evaluated in previous studies include serum CA-125 alone, in combination with other markers or in conjunction with transvaginal ultrasound (TVS) (3-6). However, large randomized trials have failed to demonstrate meaningful survival benefit (7) or a single measure of these approaches. The mortality results from the long standing United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) involving more than 200,000 participants have very recently been published (8). The overall results did not show a statistically significant reduction for multimodal screening (CA125 + intravaginal ultrasound) based on the Risk of Ovarian Cancer Algorithm (ROCA), and detection of ovarian cancers by screening in both study groups was limited (59% in the multimodal screening group and 51% in the ultrasound only group). However, secondary analyses accounting for the time lag in survival benefit estimated a reduction of 23% (95% CI, 1-46) in mortality in years 7-14 (8). Further follow up of the study participants is ongoing to confirm these findings, and a separate study in high risk patients is also ongoing. The take-home message from this UKCTOCS study is that we must and can focus on mechanisms of early cancer detection.
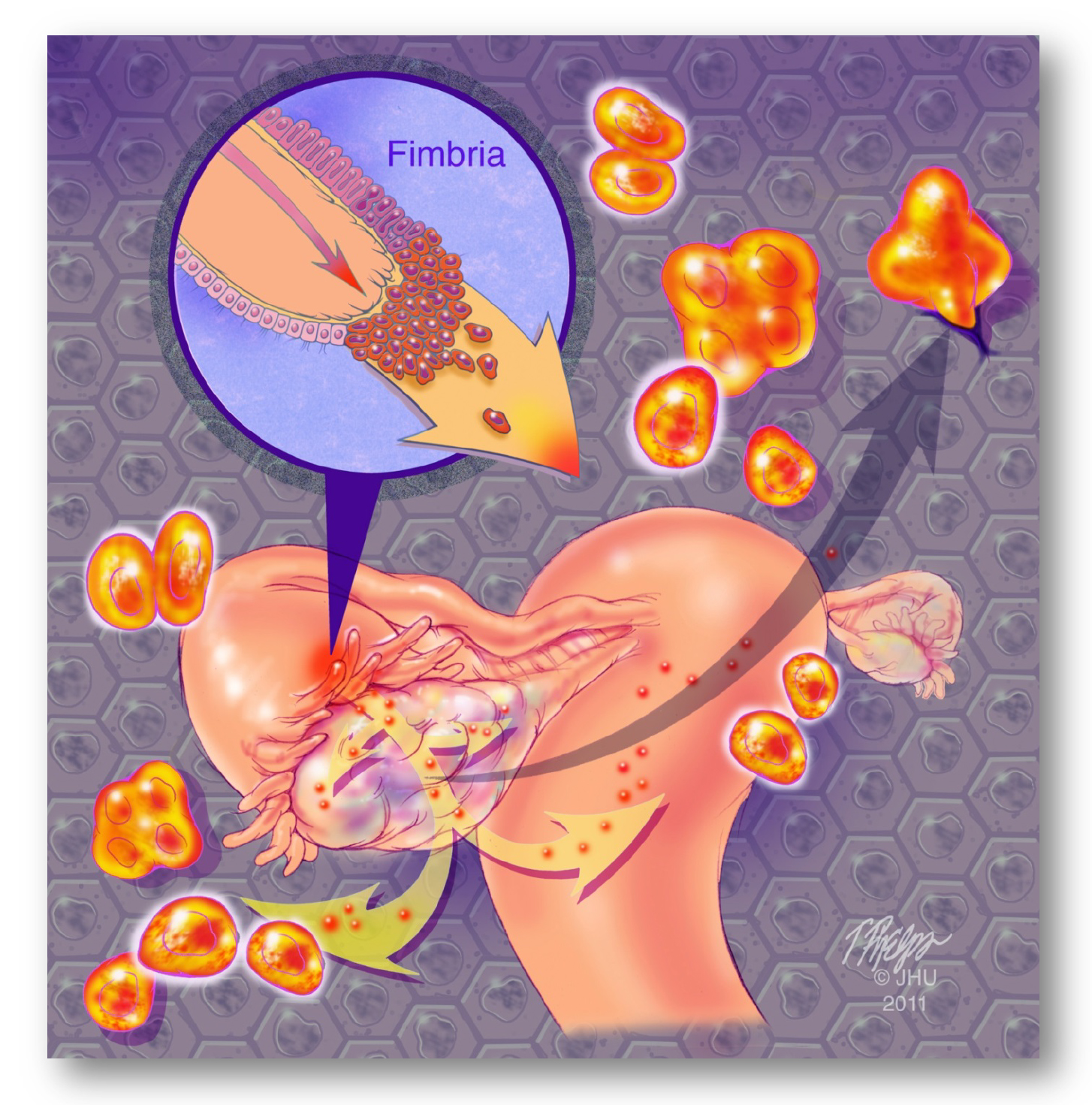
Unlike cancers arising in the colon, breast, cervix, endometrium, and prostate where the early events of carcinogenesis can be studied because of relative accessibility of these organs the fallopian tubes and ovaries are not readily accessible for direct screening. The poor survival of women with ovarian cancer has been attributed to the fact that at diagnosis about 75% of women have advanced stage disease. In contrast, women with stage I tumors have a far better prognosis, and therefore it has been argued that a true impact in reducing the burden of ovarian cancer requires detection of stage I tumors. However, almost all stage I tumors are endometrioid, clear cell, low-grade serous, or mucinous carcinomas, whereas high-grade serous carcinoma (HGSC), which makes up approximately 70% of all ovarian cancers and accounts for 90% of the deaths from this disease, are almost never stage I at diagnosis. This is in line with recent studies showingthat the earliest lesions that herald the development of most ovarian HGSCs are serous tubal intraepithelial carcinomas (STICs) and that the ovary is involved secondarily (Fig. 1 and Fig. 4; reviewed in (9)). Examining the histology of a normal ovary reveals that ovarian surface epithelium is the only epithelial cell layer which is of mesothelial origin. However, precursor lesions are exceptionally rare on the ovarian surface epithelium and all ovarian serous carcinomas exhibit mullerian rather than mesothelial phenotypes, arguing if ovarian surface epithelium is the cell of origin of ovarian serous carcinoma. Evidence to support the tubal origin of ovarian high-grade serous carcinoma is summarized in this Supplemental Figure. Based on FIGO staging, most ovarian HGSCs are stage III or IV at their inception. Moreover, this new hypothesis can also explain the occurrence of primary peritoneal "ovarian" carcinoma. Several key investigators from JHU and other institutions have contributed to this important discovery. Their research findings (10-16), along with studies by a number of other investigators (17-21), support the concept of shifting screening from disease confined to the ovary to the detection of low volume disease anywhere in the pelvis (22). Please refer to this review article for further information about the tubal origin of ovarian cancer paradigm. Further information about the precursor lesions of ovarian HGSC, please click here.
In fact, a number of studies have shown that the prognosis of ovarian cancer (especially high-grade serous carcinoma, HGSC), in addition to stage and grade at diagnosis, is crucially affected by the initial volume of disease and the amount of residual disease after cytoreductive surgery (23). In other words, size matters – detecting ovarian cancer at low volume could potentially improve ovarian cancer patient outcome (23). Recent advances in analyzing the genomic landscape of ovarian cancer by TCGA confirmed that ovarian cancer is characterized by enormous structural variations. However, other than the almost universal mutations in TP53 and common aberrations in BRCA genes, genomic variations in individual genes each tend to account for only a small fraction of the disease population. As a consequence, biomarkers associated with upstream genomic changes that are closely related to tumor initiation and tumorigenesis such as TP53 will have a very high specificity as it represents a trunk mutation. This feature provides a highly specific biomarker for detection of HGSC, since somatic mutations would in theory arise exclusively in tumor cells but not in non-neoplastic normal cells. However, the potential rarity of tumor DNA in the body fluid may limit detection sensitivity. To overcome this obstacle, scientists at JHU have recently developed a SafeSeq technology for a highly sensitive massively parallel sequencing assay using a panel of genes (including TP53) commonly mutated in gynecologic cancer (24).
Figure 4. Barrier 1. The lack of effective tools for early detection of HGSC is largely because we did not know the precursor lesions of HGSC until recent research findings proposed a new paradigm- many HGSCs are derived from serous tubal intraepithelial carcinoma (STIC) in the fallopian tube, especially the fimbriated ends. STIC cells can then shed onto ovarian and peritoneal surfaces to become an “ovarian” carcinoma.
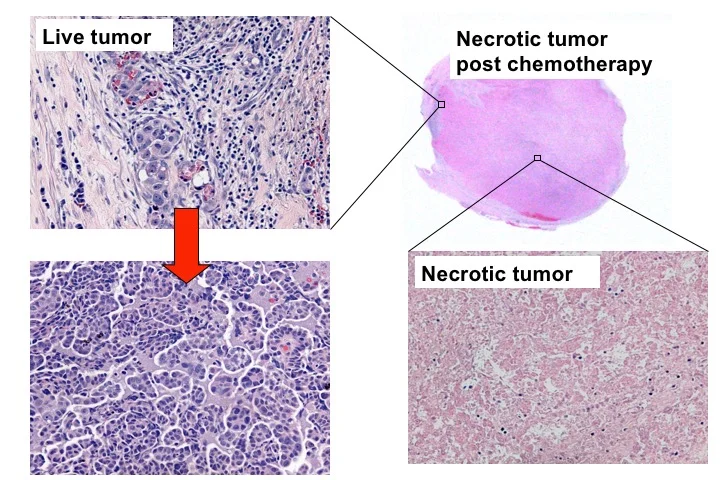
Task 2: The need to understand resistant/refractory mechanisms – Cytotoxic chemotherapy has become a standard in the treatment of advanced stage cancer. Patients with ovarian HGSC, most of whom are diagnosed with advanced stage disease, require cytoreductive surgery followed by chemotherapy treatment administrated by cycles of platinum and taxane combination therapy. Although the majority of tumors are initially responsive to chemotherapy, specific tumor cells refractory to prior therapy emerge and develop into recurrent tumors (Figure 5) (36). This leads to high morbidity and mortality for patients with advanced high-grade serous ovarian cancer (HGSC). As many as 80% of HGSC patients experience recurrence after first-line chemotherapy, and, thus, need to be treated with other chemotherapeutic agents, which are often associated with significant side effects and minimal improvement in overall disease outcome. Accordingly, understanding the mechanism leading to chemoresistance and developing an innovative strategy to treat resistant HGSC is an unmet need.
Figure 5. After chemotherapy (carboplatin and paciltaxel), the majority of ovarian HGSC cells die, forming tumor necrosis. However, tumor cells that are refractory to chemotherapy survive and expand to form a recurrent tumor. It is the recurrent chemoresistant tumor cells that cause cancer-related morbidity and mortality.
Task 3: The need to develop potent combined immunotherapy strategies – Engaging the patient’s immune system to treat cancer with immunotherapy is showing striking clinical success in some cancers that have a natural infiltration of effector T cells (28) (videos below). Ovarian cancer patients with T cell-rich tumors have a 38% chance of 5-year survival, compared to 4.5% for those with T cell-poor tumors. Furthermore, patients who undergo optimal surgical debulking and have a complete response to platinum-based chemotherapy have a 75% chance of 5-year survival if their tumor was enriched with T cells, but only a 12% chance if their tumor had few T cells (29). More than half of newly diagnosed ovarian cancer patients have tumors that are naturally infiltrated with T cells. However, even if T cells are present within a tumor, multiple regulatory signals present on both tumor cells and suppressive immune cells including regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC) within the tumor microenvironment may block T cells from exerting an effective response. Consistent with this, ovarian cancer patients with high CD8/Treg ratios survive longer than those with low CD8/Treg ratios (30, 31) (Figure 6). It has been proposed that the failure of the immune system to control ovarian cancer may result from inadequate production and/or stimulation of T cells, or from a dominant state of negative immune regulation. Several innovative approaches including tumor antigen-based immunotherapy together with immune checkpoint blockage and engineered chimeric antigen receptor (CAR) T-cell therapy have been proposed to overcome this critical barrier, which enhances the efficacy of immunotherapy in ovarian cancer. Immune-based therapy can also take advantage of the genomic alterations in neoplasms without active vaccination or transfer of reactive T cells. It has been recently proposed that carcinomas with mismatch repair (MMR) deficiency (ovarian and uterine endometrioid carcinomas) are responsive to anti-PD-1 therapy. This is because the increased repertoire of neo-antigens in cancer cells due to the MMR-induced mutation load and the release of the "brake" of immune response (immune checkpoints such as PD-1, CTLA4, IDO-1 pathways) using the checkpoint inhibitors triggers the cytotoxic immune reaction leading to killing of tumor cells (Figure 7). A case study is illustrated.
Fig. 7A. An example of immune checkpoint pathway (PD-1/PD-L1) in immune evasion in tumor cells.
Fig. 7B. PD-1 positive T lymphocytes infiltrate a high-grade ovarian serous carcinoma.
Task 4. Precision medicine for gynecologic cancer - The completion of cancer genome projects, advances in molecular technologies and emerging concepts in studying pathogenesis are reshaping gynecologic cancer research. One of the main triumphs in this endeavor is the potential introduction of biology-based therapy that can be tailored to individual women with advanced ovarian and uterine cancer. This is made possible by revealing the underlying molecular aberrations that are amenable for therapy and are unique to each cancer. As examples, inhibitors of the PARP and VEGF pathways have shown promise in prolonging progression-free survival in ovarian cancer patients. Over the past few years, the remarkable successes of immune checkpoint inhibitors and other novel immunotherapies have demonstrated their enormous potential for fighting gynecologic cancer; these therapies include tumor antigen-based immunotherapy together with immune checkpoint blockage and engineered chimeric antigen receptor (CAR) T-cell therapy. Endometrioid carcinomas with mismatch repair (MMR) deficiency are highly responsive to anti-PD-1 therapy because of the increased repertoire of neo-antigens in cancer cells due to the MMR-induced mutation load. Researchers are exploiting the fundamental vulnerability of homologous recombination (HR) deficiency in ovarian high-grade serous carcinomas. For example, in addition to BRCA1 and BRCA2, tumors harboring inactivating somatic mutations of ARID1A (a participant in HR DNA damage repair) in uterine and ovarian carcinomas are sensitive to EZH2 and PARP inhibitors. As described above, revealing the mechanisms of resistance to paclitaxel has allowed for the discovery of the roles of Spleen Tyrosine Kinase (SYK) in this process and blocking of SYK activity using small compound inhibitors has been seen to be promising to synergistically sensitize paclitaxel in ovarian cancer with SYK activation. Furthermore, clinical studies are underway to determine if Herceptin is effective to treat HER2 positive uterine serous carcinomas, and if MEK inhibitors have clinical benefit in ovarian low-grade serous carcinomas. Given the recent development indicated above, there are several challenges ahead in realizing this goal. One is Intratumoral heterogeneity, in which not all tumor cells exhibit the molecular alterations to be targeted. Moreover, functional redundancy of pathway cross-talk may “rescue” certain targeted pathways, and recurrent diseases may emerge due to Darwinian selection for the tumor clones that “recover” from single target-based therapy. Importantly, a lack of clinically valid biomarkers that can predict the efficacy of targeted therapy would greatly limit its clinical applications.
In conclusion, we have assembled a team of investigators with expertise in cancer genetics/genomics, gynecologic pathology, tumor immunology, gynecologic oncology, molecular biology, animal modeling, proteomics, and biomarker discovery. Together, we have a strong track record in pioneering the scientific discovers of ovarian cancer etiology, immune tolerance, and chemotherapy resistant mechanisms. Based on a deeper understanding of disease etiology, tumor immunology, and signaling pathways in chemoresistance, we have developed new rational strategies that encompass the spectrum of clinical management of ovarian cancer - from prevention and early detection to therapeutics for patients with invasive disease.
References Cited
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5-29.
2. Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC, Jr., Beral V, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nature reviews Cancer. 2015;15:668-79.
3. Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. American journal of obstetrics and gynecology. 2005;193:1630-9.
4. Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113:775-82.
5. Kobayashi H, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2008;18:414-20.
6. Menon U, Skates SJ, Lewis S, Rosenthal AN, Rufford B, Sibley K, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:7919-26.
7. Verheijen RH, Zweemer RP. Screening to improve ovarian cancer prognosis? Lancet. 2015.
8. Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2015.
9. Kuhn E, Kurman RJ, Shih IM. Ovarian cancer is an imprted disease: fact or fiction? Curr Obstet Gynecol Rep. 2012;1:1-9.
10. Kuhn E, Kurman RJ, Sehdev AS, Shih IM. Ki-67 labeling index as an adjunct in the diagnosis of serous tubal intraepithelial carcinoma. Int J Gyn Pathol. 2012;in press.
11. Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma- evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421-6.
12. Kuhn E, Meeker A, Wang TL, Sehdev AS, Kurman RJ, Shih Ie M. Shortened telomeres in serous tubal intraepithelial carcinoma: an early event in ovarian high-grade serous carcinogenesis. Am J Surg Pathol. 2010;34:829-36.
13. Visvanathan K, Vang R, Shaw P, Gross A, Soslow R, Parkash V, et al. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. The American journal of surgical pathology. 2011;35:1766-75.
14. Sehdev AS, Kurman RJ, Kuhn E, Shih Ie M. Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including Rsf-1 (HBXAP), cyclin E and fatty acid synthase. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23:844-55.
15. Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433-43.
16. Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih Ie M. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198:351-6.
17. Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451-6.
18. Piek JM, van Diest PJ, Zweemer RP, Kenemans P, Verheijen RH. Tubal ligation and risk of ovarian cancer. Lancet. 2001;358:844.
19. Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, et al. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985-90.
20. Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3-9.
21. Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. Am J Surg Pathol. 2007;31:161-9.
22. Kurman RJ, Shih Ie M. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151-60.
23. Nick AM, Coleman RL, Ramirez PT, Sood AK. A framework for a personalized surgical approach to ovarian cancer. Nature reviews Clinical oncology. 2015;12:239-45.
24. Kinde I, Bettegowda C, Wang Y, Wu J, Agrawal N, Shih Ie M, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5:167ra4.
25. Chang HW, Lee SM, Goodman SN, Singer G, Cho SK, Sokoll LJ, et al. Assessment of plasma DNA levels, allelic imbalance, and CA 125 as diagnostic tests for cancer. J Natl Cancer Inst. 2002;94:1697-703.
26. Wang BG, Huang HY, Chen YC, Bristow RE, Kassauei K, Cheng CC, et al. Increased plasma DNA integrity in cancer patients. Cancer Res. 2003;63:3966-8.
27. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24.
28. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252-64.
29. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348:203-13.
30. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18538-43.
31. Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer immunology, immunotherapy : CII. 2009;58:449-59.
32. Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Molecular cancer therapeutics. 2012;11:517-25.
33. Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. European journal of cancer. 2008;44:46-53.
34. Yen MJ, Hsu CY, Mao TL, Wu TC, Roden R, Wang TL, et al. Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma. Clin Cancer Res. 2006;12:827-31.
35. Hassan R, Viner JL, Wang QC, Margulies I, Kreitman RJ, Pastan I. Anti-tumor activity of K1-LysPE38QQR, an immunotoxin targeting mesothelin, a cell-surface antigen overexpressed in ovarian cancer and malignant mesothelioma. J Immunother. 2000;23:473-9.
36. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714-26.